Extraordinary thermal stability of an oligodeoxynucleotide octamer constructed from alternating 7-deaza-7-iodo guanine and 5-iodocytosine base pairs – DNA duplex stabilization by halogen bonds?
Natalya Ramzaeva, Henning Eickmeier, Helmut Rosemeyer
Chem. Biodiversity 2014, DOI:10.1002/cbdv.201300300
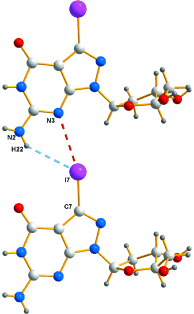
A reinvestigation of the published X-ray crystal-structure analyses of 7-halogenated (Br, I) 8-aza-7-deaza-2’-deoxyguanosines Br7c7z8Gd; 1a and I7c7z8Gd, 1b, as well as of the structurally related 7-deaza-7-iodo-2’-deoxy-ß-D-ribofuranosyladenine (ß-I7c7Ad; 2-6e in Table 1) and its α-D-anomer (α -I7c7Ad; 3) clearly revealed the existence of halogen bonds between corresponding halogen substituents and the adjacent N(3)-atoms of neighboring nucleoside molecules within the single crystals. These halogen bonds can be rationalized by the presence of a region of positive electrostatic potential, the σ-hole, on the outermost portion the halogen’s surface, while the three unshared pairs of electrons produce a belt of negative electrostatic potential around the central part of the halogen substituent. The N(3) atoms of the halogenated nucleosides carry a partial negative charge. This novel type of bonding between nucleosides was tentatively used to explain the extraordinary high stability of oligodeoxynucleotides constructed from halogenated nucleotide building blocks.